- 接合関連
「接着」の基礎知識——接着剤と粘着剤の違いから学ぶ
接着と粘着の大きな違い
「接着」と「粘着」。日常でもよく聞く言葉ですが、その正確な違いについて、ご存知でしょうか? 国語辞書では、接着が「ぴったりとくっつくこと」、粘着は「ねばりつくこと」と定義されています。
デクセリアルズが生産する「接着剤」と「粘着剤」について、いずれも物と物をくっつける(固定する)機能については同じです。粘着も「接着」の一種に分類されますが、強力な固定を実現する接着剤に対して、緩やかに固定できるのが粘着剤という違いがあります。これを別の言葉で言い変えるならば、「剥がれてはいけないのが接着」であるのに対して「剥がすことができるのが粘着」と定義できるでしょう。
Aという物とBという物を接着した場合、それを無理に剥がそうとすると、最終的にAまたはB、もしくは両方の物が壊れてしまいます。それに対して粘着の場合には、剥がそうとしたとき、AとBの間で二つをくっつけている粘着剤は壊れますが、AとBは壊れずに元の状態のままで、再利用することができます。
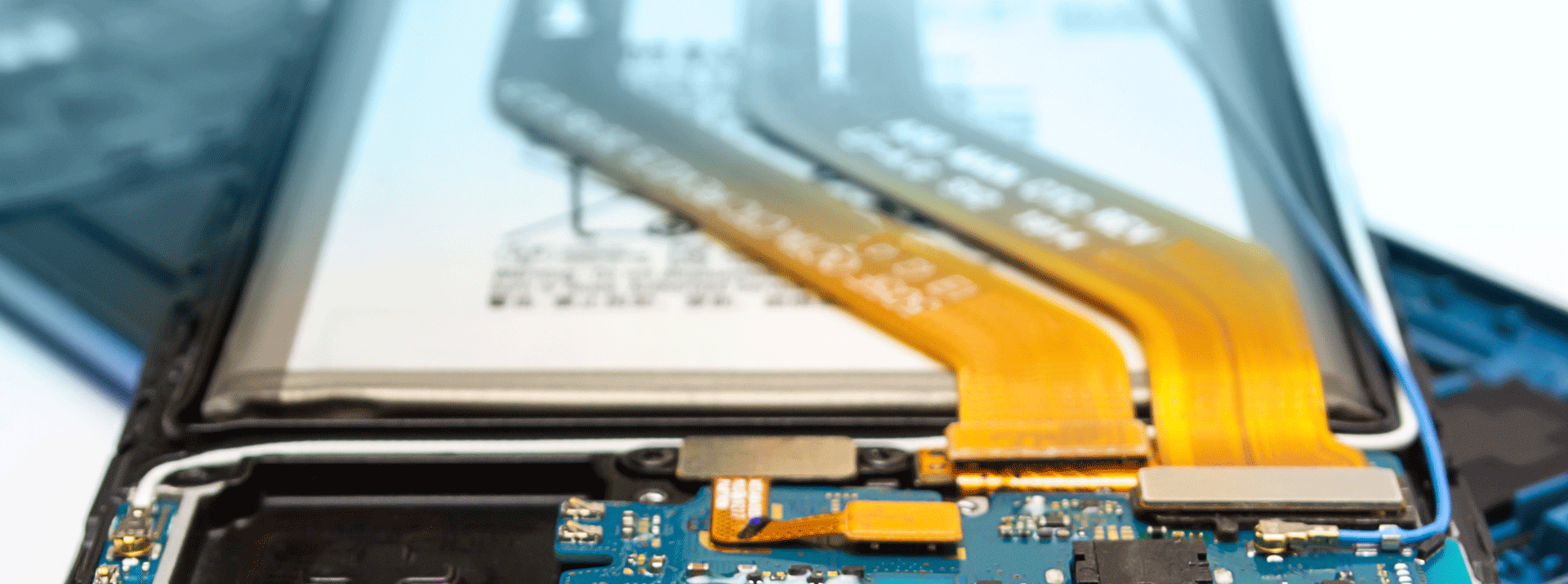
スマートフォンのタッチパネルなどの部品が、接着剤ではなく、粘着剤で固定されているのは、この特性を活かすためです。粘着剤を用いれば、修理の際に一度部品を剥がした後でも、再び粘着剤を使うことで、元通りに修復することができます。一方、基板などの剥がれてはいけない部品の固定においては、強度が求められるために接着剤が用いられます。(別記事「接着剤の基礎知識(その物性について)」では、接着剤の液状特性(チキソ性・粘土)や硬化物特性(弾性率)について解説しています)
接着剤、粘着剤がものをくっつけるメカニズム
接着剤と粘着剤では「物がくっつくメカニズム」が異なります。一般的に反応性の接着剤は、元の状態から加熱や紫外線などのきっかけによって、化学変化を起こすことで物体を接着します。それに対して粘着剤は、そのもの自体が粘り気と弾性を持つ物質であり、物をくっつけた後もその状態で安定しています。粘着剤は、水、溶剤、熱などを使用せず、常温で短時間、わずかな圧力を加えるだけでくっつくのが特徴です。
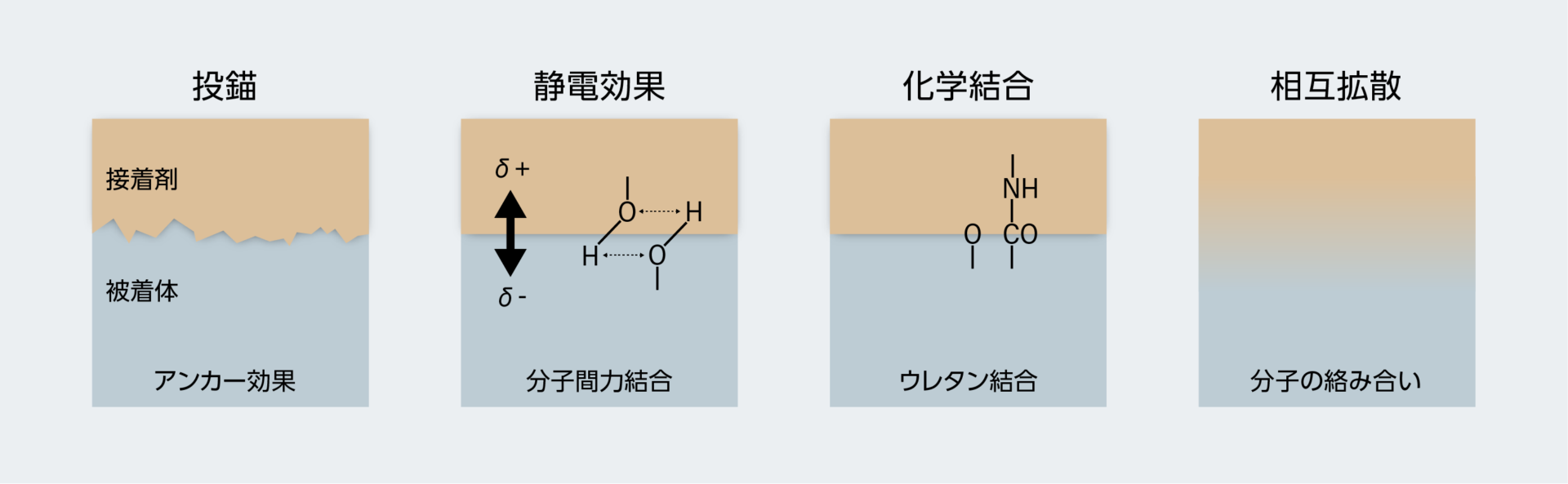
接着剤が物同士をくっつけるとき、その界面には次に挙げる4つの変化が起きています。
- 投錨効果(アンカー効果) 平らに見える物も、ミクロな世界では小さな凹凸があります。その凹凸に接着剤が入り込み、固まることで接着します。
- 静電効果 被着体同士の電気的な偏りによって、静電気の働きで接着します。プラスチックの下敷きをこすって髪の毛に近づけると静電力で引きつけられ逆立つ現象と同様の原理です。
- 化学結合 被着体同士の界面で、分子が科学的に結合することで接着が行われます。エポキシ系などの硬化系接着剤がこの原理を利用しています。詳しくは別記事「熱硬化型接着剤のメカニズム(架橋反応とその種類)」「紫外線でも熱でも固まるエポキシ系接着剤の技術」で解説しています。
- 相互拡散 被着体の表面を溶かして、分子を絡ませて固まらせることで接着します。プラモデルなどに使われる、溶剤が主成分の揮発性接着剤がこの原理を利用しています。
メカニズムの違いから、接着剤と粘着剤は、それぞれに得意な使用環境、使用目的があります。粘着剤は、テープ状、フィルム状に加工することで、連続的に平滑な物同士をくっつけることを得意としています。それに対して液状の接着剤は、凹凸がある場所や、狭い場所にも入り込んで硬化できることから、さまざまな形状の物同士の接着に適しています。
接着剤は「厚み」のあるほうが接着力が強い
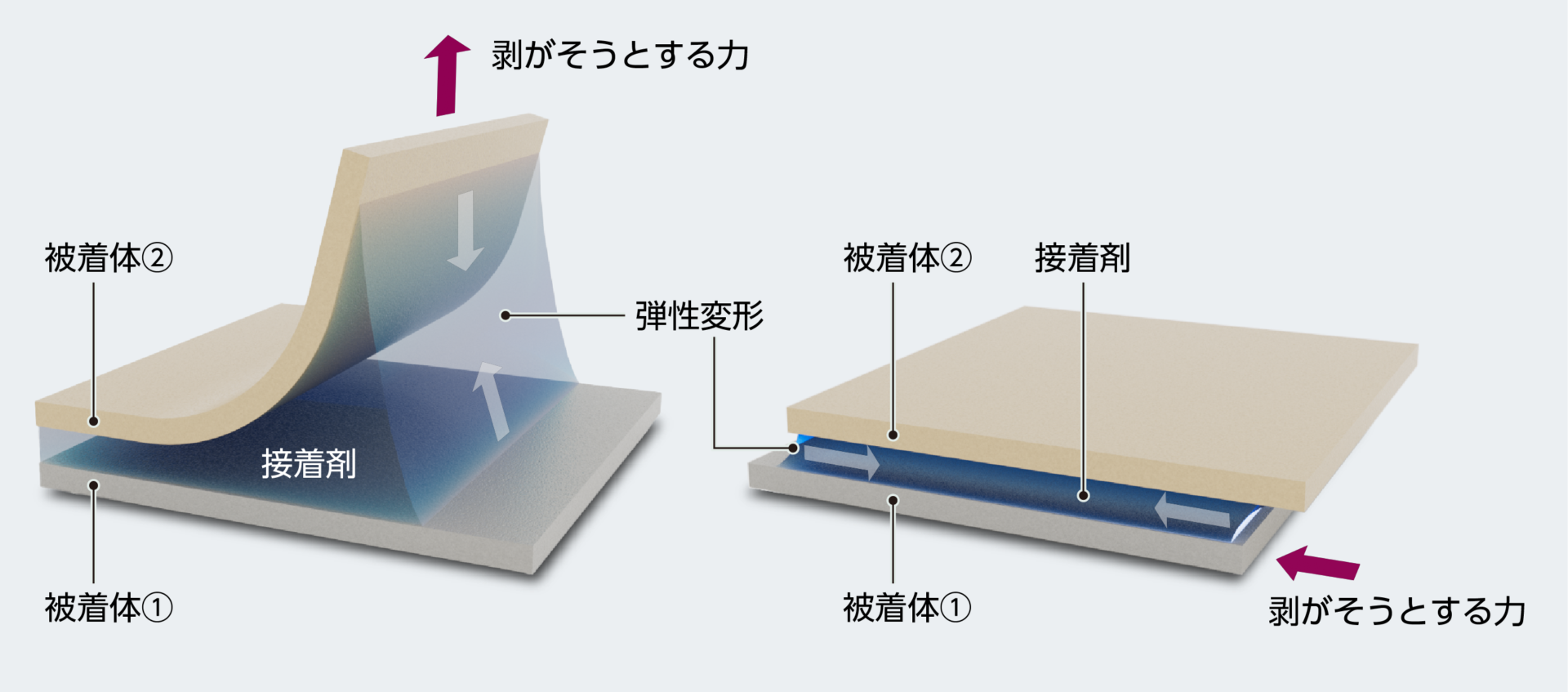
「剥がれてはいけない」場面で用いられる接着剤は、界面の接着力だけでなく、接着剤全体の物性によって剥がそうとする力に抵抗しています。接着した物に何らかの力が加わったとき、硬化した接着剤の樹脂全体が、剥がそうとする力の一部を変形(或いは弾性変形)という形で吸収し、剥がれを阻止しています。そのため、硬化した接着剤全体(バルク)の厚みがある方が変形量を大きくでき、結果として大きな接着力を出せることになります。よって接着剤を用いる場合、固まった後の樹脂の弾性を上手く設計することが、必要な接着力を得るために大切となるのです。
上手な接着には「表面処理」が大切
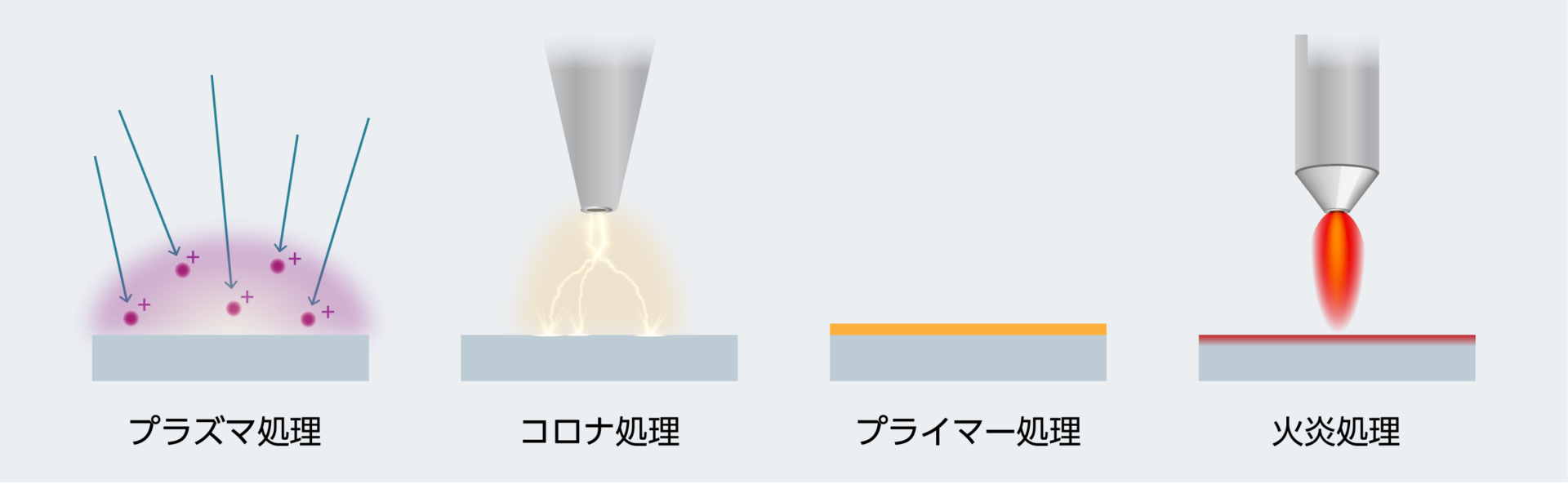
接着のためには、使用する接着剤や粘着剤だけでなく、接着する物同士の表面を「くっつきやすく」しておくことがとても重要です。液状の接着剤は被着体に塗布すると水滴のように広がりますが、接着する面積が大きいほうが接着力が高まるため、界面の「濡れ性」が高い物質は接着しやすくなります。テフロンやシリコーン、ポリエチレン、ポリプロピレンなどの物質は液体を弾く性質を持っているため、接着するのが難しい素材となります。また、接着剤は硬化した際に、表面が粗く凸凹がたくさんあるほうが投錨硬化(アンカー効果)が高まります。そのため接着の現場では、被着体を放電や火にさらすことで界面を粗くするなどの、さまざまな表面処理が行われています。
- プラズマ処理 アルゴン、ヘリウム、窒素、酸素などのガスイオンで表面を洗浄したり、官能基(アミノ、カルボキシル、ヒドロキシル、アルデヒド)を生成したりする方法
- コロナ処理 コロナ放電に曝し、官能基を生成とともに粗面化する方法
- プライマー処理 下地処理剤を塗布する方法(被着体に合わせて選定する必要あり)
- 火炎処理 表面を火に曝して官能基を生成する方法。ポリオレフィン等で使われる
上手に物と物を接着するためには、使用環境、使用目的、素材などに応じて最適な接着剤や粘着剤を選定するとともに、被着体も表面処理等の事前準備を行っておくことがとても大切なのです。
関連記事

私たちデクセリアルズはデバイスの進化に欠かせない材料や次世代のソリューションを生み出す、マテリアルメーカーです。
電子部品、接合材料、光学材料をはじめと世界中のパートナーと新しい価値を生み出していきます。
- SHARE
当社の製品や製造技術に関する資料をご用意しています。
無料でお気軽にダウンロードいただけます。
お役立ち資料のダウンロードはこちら
当社の製品や製造技術に関する資料をご用意しています。
無料でお気軽にダウンロードいただけます。
お役立ち資料のダウンロードはこちら