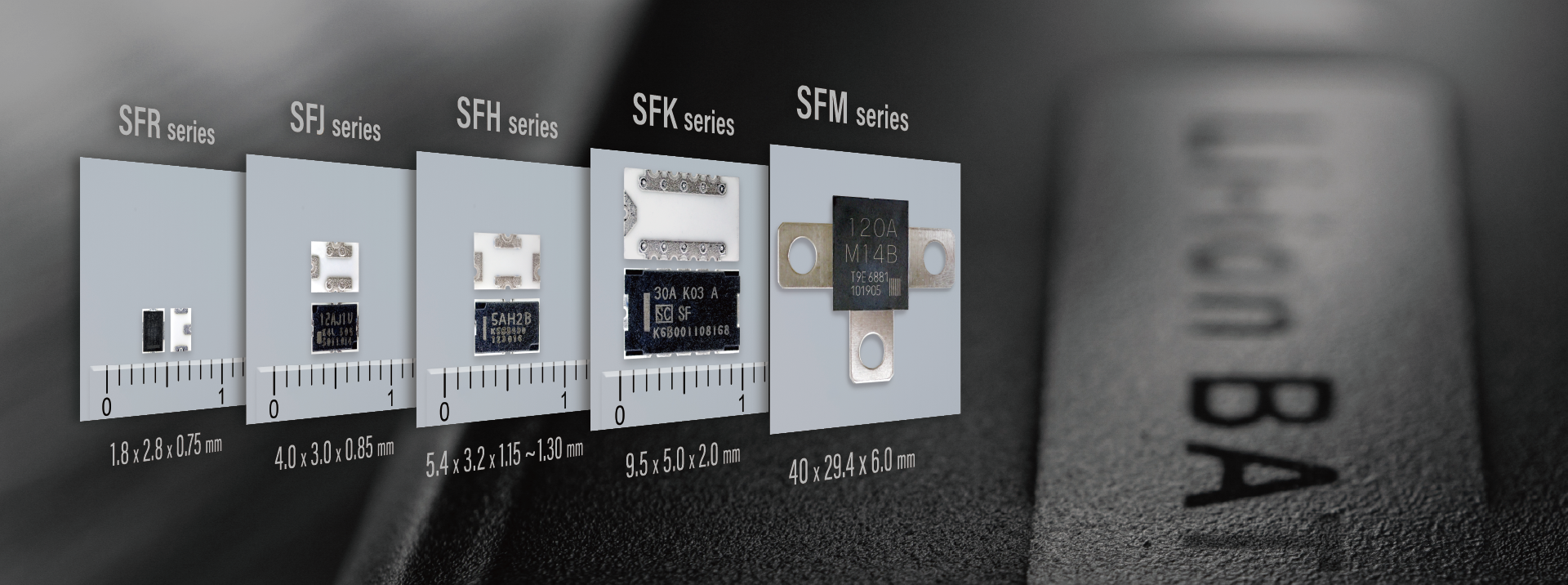
- Electronic Components
Heat and the basics of thermal management
Contents
Why thermal protection is important in electronics
Smartphones and laptops have undoubtedly become an integral part of our lives. Users may notice their smartphones heating up or the laptop’s fans constantly running to cool down after using for a long period of time.
In designing such state-of-the-art electronic devices, it is important to take into consideration heat dissipation and other thermal management measures to prevent heat-related malfunction and damage such as low-temperature burns caused by prolonged use.
As computer capabilities increase with each new generation, the energy consumption of central processing units (CPUs), the brains of computers, increases as well. The latest CPUs can consume as much as 300W of power, with most of the electrical energy eventually dissipating as heat.
The energy consumption of circuit boards used in communications base stations is also increasing, with each board consuming more than 1,000W. If nothing is done, the boards can become as hot as a hot plate. And that’s just for one board – up to 40 boards are used in an entire rack, generating enormous amount of heat. As the cost of cooling continues to rise, it is becoming ever more important to design equipment using materials and structures that are easy to cool.

Heat is generated from the motion of the particles present in all matter
In order to explain what is required for the thermal management of electronic equipment, this article will first explain the basics of heat.
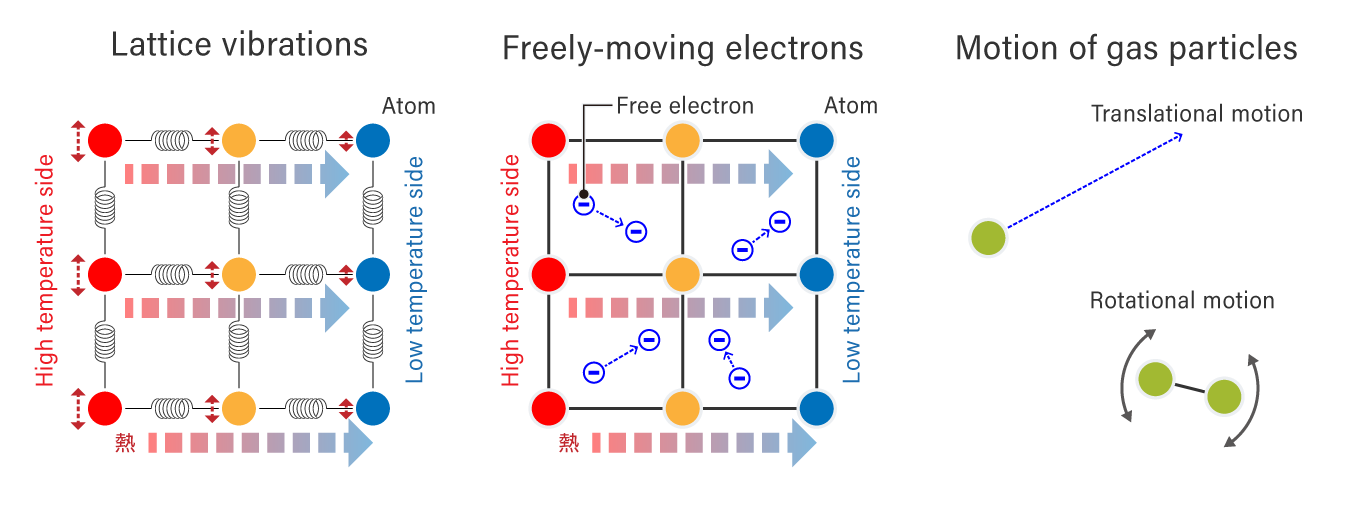
As you may remember learning in your high school physics class, heat is generated by motion of particles (molecules, atoms, and electrons) that are present in all matter. Unless the temperature is absolute zero (0K = -273.15℃), the particles of matter are in motion and have heat in proportion to their momentum. (To be precise, even at absolute zero, atoms are undergoing slight oscillations called “zero-point energy” due to the uncertainty principle).
The motion of particles depends on the arrangement of the atoms in the substance.
Lattice vibrations occur in insulating solids such as diamonds, while a combination of freely moving electrons and lattice vibrations occurs simultaneously in solid metals. In gasses, particles are in translational and rotational motion.
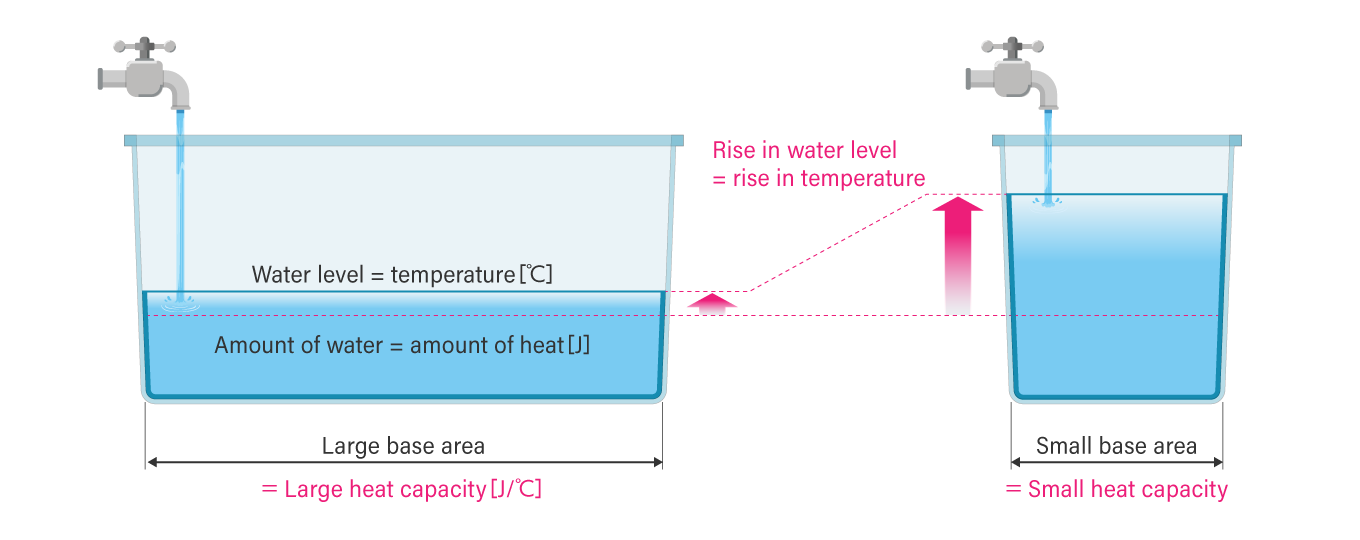
“Water in a tank” analogy to understand heat and temperature
An easy way to understand a substance’s heat capacity and temperature is to imagine water stored in a tank. The amount of heat in a substance corresponds to the amount of energy stored in that substance. In other words, just as water accumulates in a tank, energy (heat) is also stored by continuously giving it energy.
Its temperature can be compared to a tank’s water level. If energy is given to a substance with a large heat capacity, its temperature (water level) does not rise as easily as water stored in a tank with a large base area. However, when the same amount of energy is added to a substance with a small heat capacity (tank with a small base area), the temperature rises quickly.
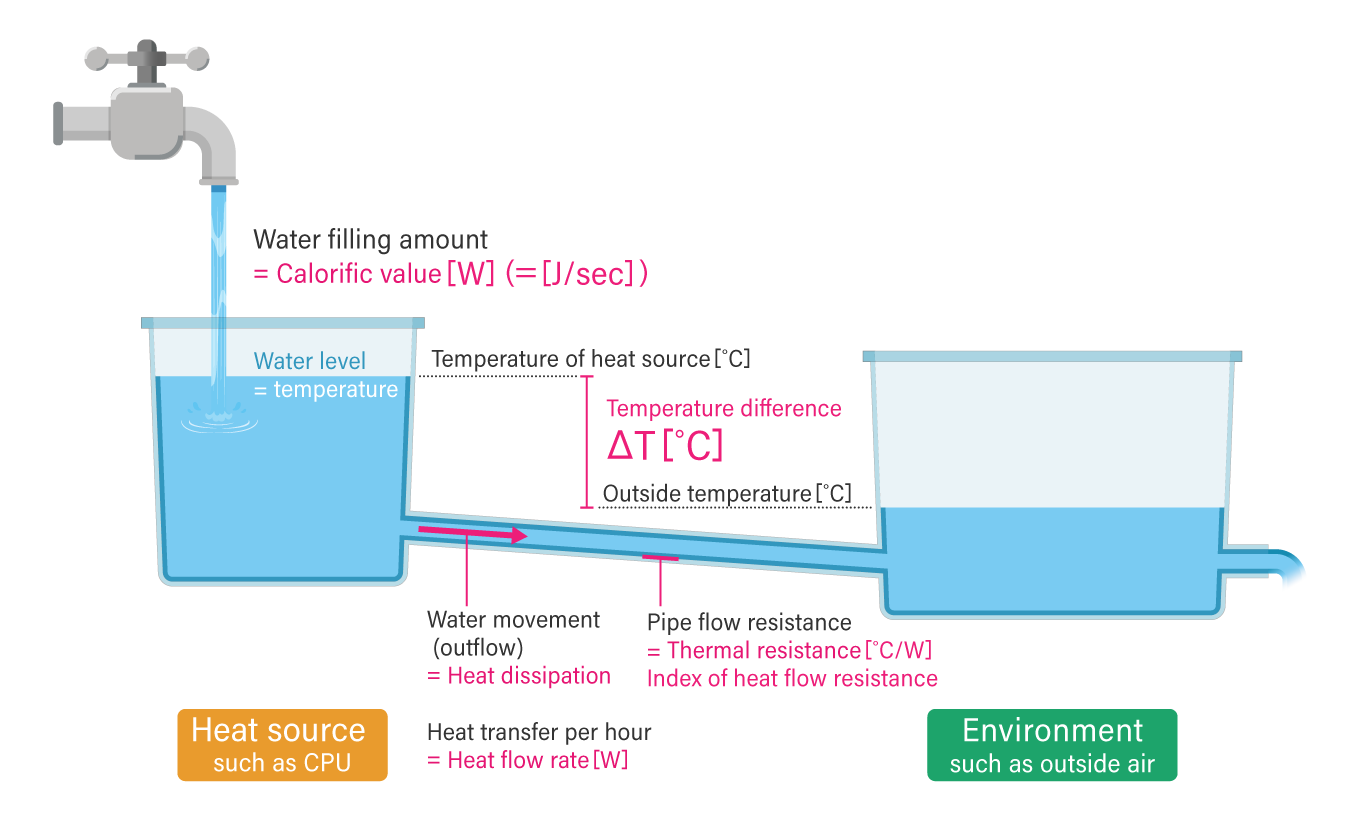
Comparing heat dissipation to water flow
To re-use the analogy of water stored in a tank, heat dissipation, where heat is emitted from an object, is similar to water flowing out of a tank. Just as water flows from a high point to a low point, heat will always flow from hot to cold unless it receives energy from the outside.
There are two things to note when considering heat generation measures. The first is the temperature difference (ΔT) between the CPU (or other heat sources) and the outside air temperature. The second is the amount of heat per hour (i.e. heat flow rate (J/sec or W)) rather than the total amount of heat (joules). The heat flow rate depends on the value of the thermal resistance of the pipe connecting the tank with water (i.e. the heat source, such as a CPU) to another tank (i.e. the outside temperature).
Common assumptions regarding heat dissipation
As noted earlier, ‘0°C’ cannot be used as a reference point for relative heat. For example, when the temperature of a heat source goes from 50°C to 100°C, it would be a mistake to assume that the temperature doubles. This concept would be correct if 0°C was the standard reference point. However, 0°C is merely the freezing point of water. What should be considered is the temperature difference between the heat source and the target cooling temperature. If the temperature of a heat source rises from 50°C to 100°C and there is a need to cool it down to an ambient temperature of 25°C, there is a temperature difference of 75°C. In other words, when it rises from 50°C to 100°C, the correct thinking is not that the temperature doubles, but rather that the temperature difference to airincreases by 75°C, or three times higher than 25°C. Therefore, what is important in heat dissipation is in fact the difference between the ambient temperature and the heat source.
- SHARE
 Back to top
Back to top  Contact us
Contact us